Patient distrust in hospitals and corporations to protect their personal data is a challenge to coll...

Enter Search Inquiries Below

Patient distrust in hospitals and corporations to protect their personal data is a challenge to coll...

Hospitals are still understaffed after the COVID-19 pandemic and are always looking for ways to stre...

Breakthroughs in health tech and patient care are irrelevant if patients can’t use them. We must enh...

Artificial intelligence (AI) and interconnected technologies are increasingly accepted in business a...

We are collecting a lot more health data today than we have in the past, but turning all those data ...


Integrated care is vital to patients, meaning we need systems that communicate with each other so pr...



You might have seen the memes that point out 2022 is pronounced the same way as “2020, too.” It’s hu...

Enterey Consulting focuses on the 5 key characteristics of go-to Life Science Consultants and Projec...

Last year, Enterey Consulting showcased a series of blogs covering the most important characteristic...

In 2020-21, Enterey Consulting conducted a survey to determine the character traits of the highest p...

Sam Howard is the Senior Program Manager at Dendreon Pharmaceuticals, one of the world’s leading imm...

There are many different components that contribute to disengagement in a project management office....

Complacency is a hurdle that appears in many different organizations. Over the past year and a half,...

Good management is a key component of a company within any industry. With a strong, trusted manageme...

It is no secret that disengagement in a project management office leads to several negative outcomes...

An engaged workforce can drive a company's success. Unfortunately, recent research in a study by Gal...





Compliance protocol in the medical device manufacturing industry isn’t just extensive and complex — ...

Pharmaceuticals companies in every life science vertical have always prioritized risk management to ...

Single-use technologies in biopharmaceutical manufacturing have had a major influence in 2020 and 20...

An effective and efficient Project Portfolio Management (PPM) process delivers the framework life sc...

At Enterey Consulting, our team of innovative life science consultants knows firsthand that project ...

Resource allocation plays a critical role in helping life science companies remain aligned with over...


Over the last several weeks, the Enterey team has focused on the key characteristics of go-to life s...

Over the last several weeks, Enterey Consulting has showcased the most important characteristics our...

The importance of proficiency as a key character trait for life science go-to consultants can’t be o...

Self-motivation can be challenging to harness. Some days you wake up firing on all cylinders, full o...

Mastering a positive mindset is a key characteristic of go-to life science consultants. However, a p...



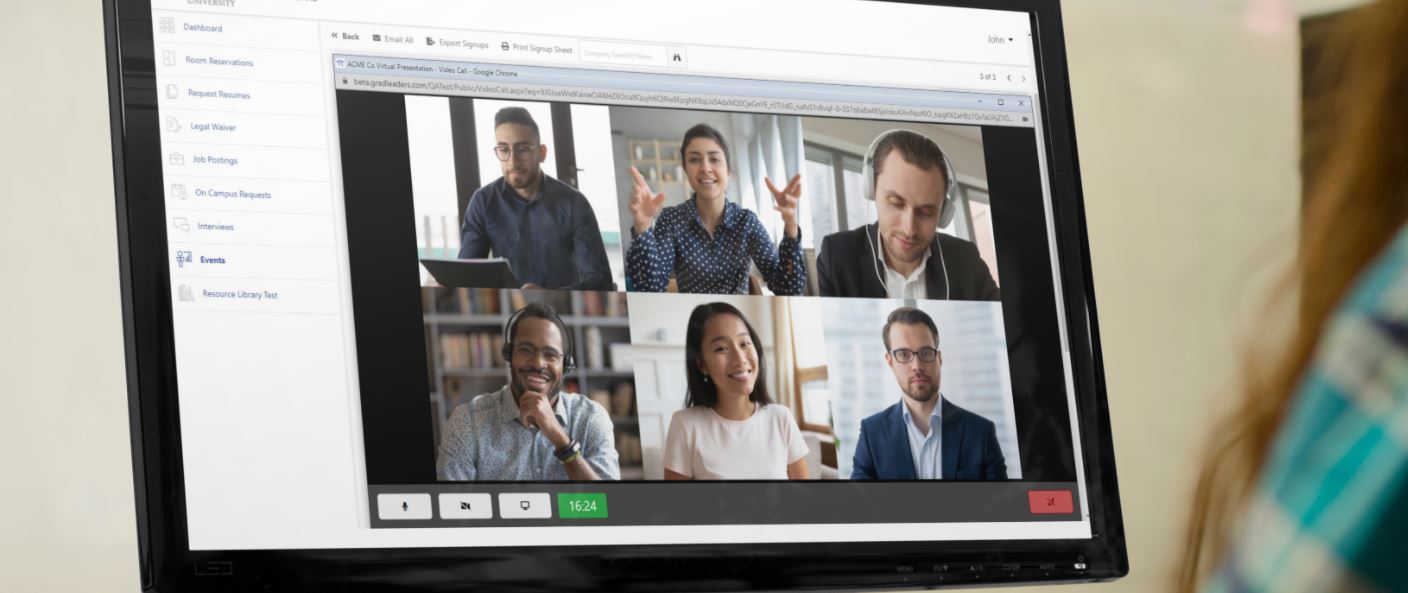






NOTE: Master Control GxP Lifeline first published this article on July 29, 2020. Click here to read ...

Resource allocation practices equip life science leaders to systematically plan, manage, and assign ...

Life science organizations have always encountered relentless project disruptions on both an interna...






NOTE: Master Control GxP Lifeline first published this interview with Enterey President and CEO, Mik...



A cohesive life science operational plan plays an integral role in the success of virtually any life...

Life Science Management With Single-Use Technology The primary considerations for any pharmaceutical...

Successful life science organizations across virtually every vertical must continuously recalibrate ...

Most life science companies experience a missed project milestone or deliverable at some point. Biot...

The COVID-19 global pandemic has had a significant impact throughout the life sciences industry, inc...

Life science organizations across every functional area rely on life science consulting firms to boo...

Effective life science project management documentation practices play a vital role in driving effic...

Process inefficiencies and redundancies can wreak havoc on a life science organization's technical o...

The identification of COVID-19 coronavirus has made an impact on our economy, driven by the challeng...

A new world is emerging. Not only is climate change creating new weather patterns the world over and...

The Medical Device Industry and the Dark Web

According to the Society for Human Resource Management (SHRM), “[No] company, small or large, can wi...

Avalon GloboCare and GE Healthcare Announce Strategic Partnership to Accelerate Standardized Automat...


COPYCATS WANTED: PFIZER, AZ AND MORE UNDER THREAT AS CHINA SOLICITS GENERICS


At Enterey we are focused on using our strengths and values to drive successful transformation proje...
Online via GoToWebinar

By 2020, approximately 90% of prescription drugs were serialized worldwide (Source: R&D). By 2023, l...


Irvine, California 30 years ago, when small-molecule generic drugs first entered the market, they ch...

Irvine, California

